


Electrolysis
Reflections and Exam tips
What you need to know:
-Electrolysis (electro~ lysis) is the breaking down of a compound containing ions, into its elements using electricity.
-Ionic substances allow electric currents to flow through them when they are molten or in solution.
-Ionic compounds contain positive (cations) and negative (anions) ions.
-The molten compound or solution is called an electrolyte.
During electrolysis:
-Cations move towards the negative electrode (cathode) and become reduced by gaining electrons
-Anions move towards the positive electrode (anode) and become oxidised by losing electrons.
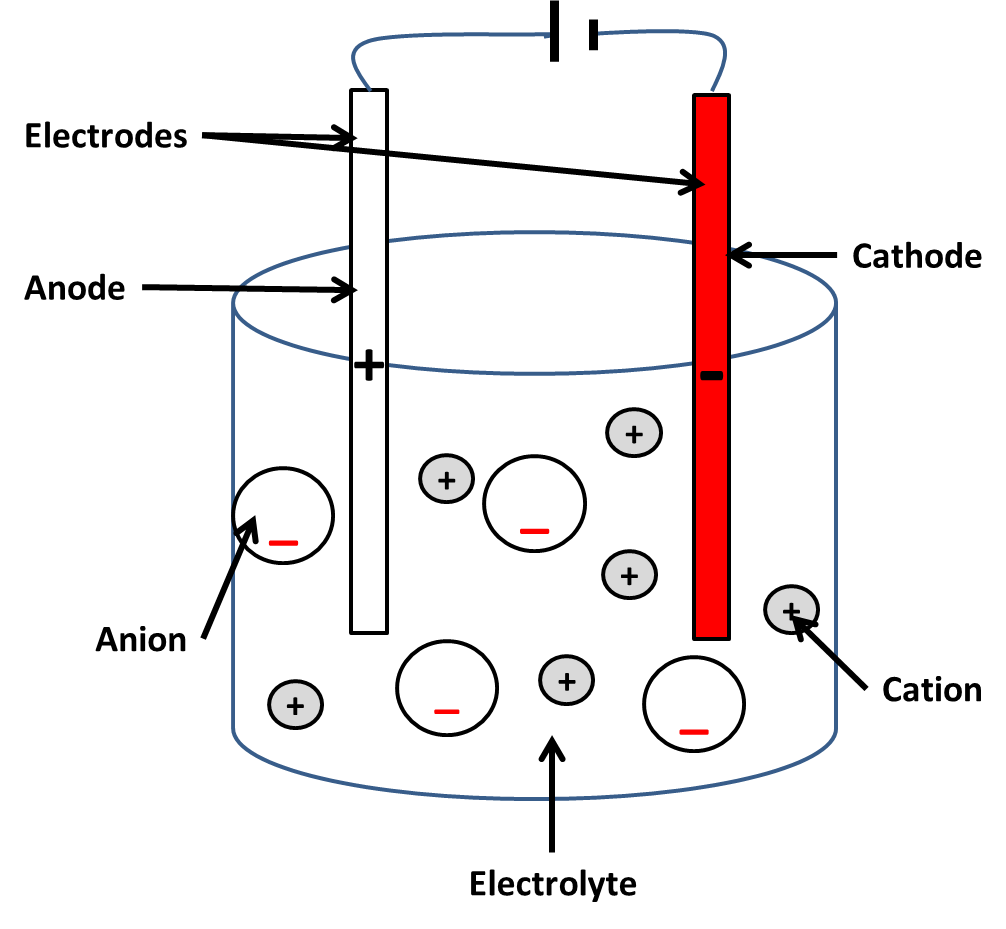
During electrolysis a positive ion is attracted to the cathode, it gains electrons, to become a neutral atom. This process of gaining electrons is called reduction, and the ions have been reduced. Ions with a single positive charge (e.g. Na+) gain one electron, and ions with a 2+ charge (e.g. Cu2+) gain two electrons.
At the positive electrode (anode) negative ions lose electrons to become neutral atom. This is called oxidation and involves the loss of electrons. Non-metal atoms combine to form molecules.
*A redox reaction is where both reduction and oxidation occur. This can be remembered by using the word OILRIG (Oxidation Is Loss of electrons and Reduction is gain of electrons) or LEO and GERi (Loss of electrons= Oxidation, Gain of electrons = Reduction).
Half-equations
The changes happening at the different electrodes with a special type of equation called a half equation. For example, the electrolysis of sodium chloride produces the following products, chlorine gas, hydrogen gas and sodium hydroxide.
Electrolysis of brine (concentrated salt/sodium chloride solution)
At the anode:
Chloride ions are oxidised to form chlorine molecules. Chloride ions are discharged as they are less reactive than hydroxide ions.
2Cl- ——> Cl2 + 2e-
At the cathode:
Hydrogen ions are reduced to hydrogen molecules. Hydrogen is discharged as it is less reactive than sodium.
2H+ + 2e- ——> H2
Sodium (Na+) and hydroxide (OH-) ions remain in solution to produce sodium hydroxide.
-Sodium hydroxide is a strong alkali and has many industrial uses such as making soap, making paper, making bleach and neutralising acids (controlling pH).
-Chlorine is used to kill bacteria in drinking water and swimming pools. Chlorine is also used to make bleach, disinfectants and plastics.
-Hydrogen is used to make margarine as well as making hydrochloric acids.
Aluminium electrolysis
Aluminium ore (bauxite) is heated (with cryolite to reduce the melting point) until it melts. Aluminium oxide has a higher melting point and cryolite helps reduce the energy cost as the melting point is lowered to around 900°C.
Electricity is passed through the molten ore, which splits the aluminium oxide into 2 charged particles (positive aluminium & negative oxide).
The negatively charged oxide move to the positively charged carbon anodes where they are oxidised. The oxygen produced reacts with the carbon anodes to produce carbon dioxide. Over a period the anodes must be replenished.
2O2- ——> O2 + 4e-
The positively charged aluminium moves to the negatively charged cathode where are reduced.
Al3+ +3e- ——> Al
Aluminium sinks to the bottom and is collected off.
Understand that ionic compounds in the molten (l) state or dissolved have ions (aq) that are free to move around
Know that passing an electric current through a molten solution containing ions will make the elements of the ions involved
Be able to write ionic equations to show this process
Be able to explain why the electrolysis process can occur in molten substances but not solid substances
Be able to explain and give reactions for the extraction of aluminium from aluminium oxide using electrolysis
Be able to explain and give reactions for the production of chlorine, hydrogen and sodium hydroxide in the electrolysis of brine
Be able to predict the products of an electrolysis reaction based on the reactants involved
Be able to write ‘half equations’ to show the gain or loss of electrons from a chemical species in an electrolysis reaction